Last updated on April 5th, 2024 at 05:51 pm
Li-ion batteries are being used in every device now. From mobile phones to electric vehicles, numerous types of devices are powered by this excellent battery technology. In this article, we will discuss lithium-ion batteries.
We will see the history and evolution behind this technology, the battery chemistry, the life of these batteries, its advantages, disadvantages, and applications.
Table of Contents
The complete timeline of Li-ion batteries

The lithium-ion battery is one of the most popular batteries today. This widespread acceptance is due to the numerous advantages and features they exhibit during several applications. It is quite obvious that this success is a result of a series of discoveries, modifications, and other efforts. Although the journey up to the latest lithium-ion batteries has numerous stages to discuss, we will see only the important milestones here.
- 1817: Arfwedson and Berzelius discovered lithium from the petalite ore (LiAlSi4O10). The element was first successfully isolated from the ore by Brande and Davy in 1821.
- 1970: British chemist M. Stanley Whittingham proposed the first lithium battery. He also came up with the method to store lithium ions within the layers of a disulfide material. Titanium sulfide and lithium metal were used as electrodes. But the model had several limitations.
- 1973: Adam Heller proposed the lithium thionyl chloride battery which is still used in implanted medical devices and defense systems. It had a greater shelf life, high energy density, and tolerance for extreme operating temperatures.
- 1979: Ned A. Godshall, John B. Goodenough, and Koichi Mizushima demonstrated a rechargeable lithium cell with voltage in the 4V range using lithium cobalt dioxide as the positive electrode and lithium metal as the negative electrode.
- 1980: Invention of lithium graphite electrode by Rachid Yazami, which is the most commonly used electrode in commercial lithium-ion batteries from 2011.
- 1985: Akira Yoshino improved safety by assembling a prototype cell using carbonaceous material where the lithium ions and lithium cobalt oxide were used as electrodes.
- 1990: Jeff Dahn and two colleagues reported reversible intercalation of lithium ions into graphite in the presence of ethylene carbonate solvent. This finding led to the modern lithium-ion battery.
- 1991: Sony and Asahi Kasei released the first commercial lithium-ion battery.
- 2010: The global lithium-ion battery production capacity reached 20 gigawatt-hours. By 2016, it was 28 GWh, with 16.4 GWh in China. Production in 2023 is estimated by various sources to be between 400 and 1,100 GWh.
- 2011: Lithium-ion batteries accounted for 66% of all portable secondary battery sales in Japan.
Battery Chemistry
Let’s have a look at the chemistry of lithium-ion batteries.
Components
Lithium-ion batteries themselves use different combinations of electrodes. The most common combination among them is lithium cobalt oxide as cathode and lithium-doped graphite as the anode. An organic compound called ether is typically used as an electrolyte. The electrolyte consists of a huge amount of lithium salts in it.
Structure
The basic structure of a lithium-ion battery is shown in the figure below.
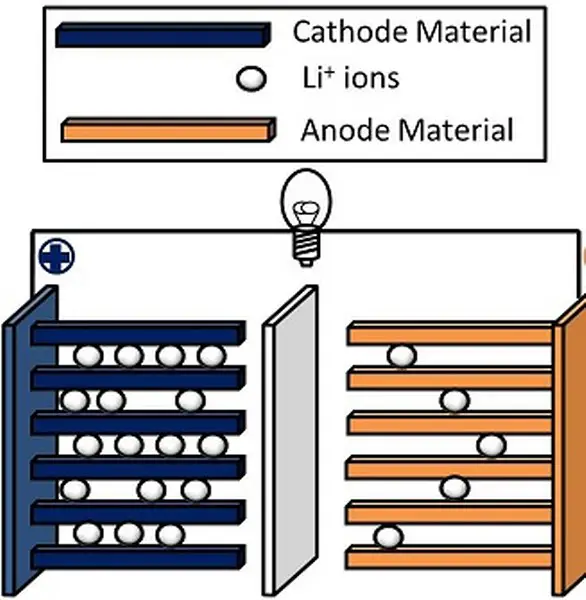
It consists of two half cells with a separator placed in between. One-half cell is the cathode and the other one is the anode. Both the half cells are filled with the electrolyte.
How does a battery work?
It works by involving two processes: Charging and discharging.
The discharging process:
The process starts with the anode. Once the anode comes in contact with the electrolyte, lithium salt in the electrolyte breaks up into carbon, lithium-ion, and electron. This lithium-ion tries to move to the cathode along with the electrons.
Note: The lithium-ion travels through the electrolyte and the separator, while the electrons reach the other side only through an external medium.
Thus, when the battery is connected to an electronic circuit, the electrons move through the circuit. As soon as the electron starts moving, the lithium ions will also move to the other side. Both the particles will then combine with the cathode to form lithium-cobalt-oxide.
The charging process:
The lithium-cobalt-oxide is a very crucial component in the lithium-ion battery as it can easily get back to cobalt, lithium ions, and electrons while charging. As we connect the battery to a charger, the reverse process happens.
It pulls the electron from the cathode and pushes it to the anode. It then combines back and returns to the lithium-doped-graphite. When the anode recovers all the ions and electrons released during the discharge phase, the battery reaches its full charge. Read more.
Energy density
The energy contained in the battery relative to its weight is known as the energy density of a battery. It is typically represented in Watt-hours per kilogram (Wh/kg). The volumetric representation of energy density is given in Watt-hours per liter (Wh/L).
The battery with a higher energy density has a longer battery run time and vice versa. Thus energy density is a crucial parameter for every battery.
The energy density of lithium-ion batteries is in the range of 90-160 Wh/kg. The average volumetric energy density of lithium-ion batteries as per the statistics of 2008 was 55 watt-hours per liter.
This has further increased to 450 watt-hours per liter by the year 2020. The progress from the year 2008 to 2020 is shown in the graph below.
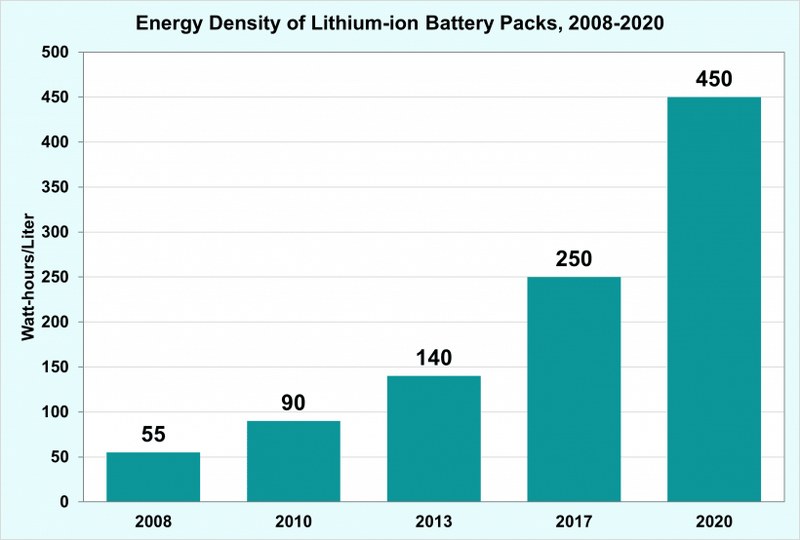
One of the highlighted applications of lithium-ion batteries is in electric vehicles (EV). The high volumetric energy density of lithium-ion batteries made it the perfect choice for EVs. The advantages of high energy density include running longer distances at the smaller battery packs, and less battery space, cost, and weight.
Battery capacity
It determines the maximum energy delivered by a battery under specified conditions. It is also the charge stored by the battery which is the mass of active material of the battery. It is represented in Ampere hours (Ah). The maximum capacity of a lithium-ion battery is recorded as approximately 3.8 Ah.
What is the life of a lithium-ion battery?
The lithium-ion battery has a life of 2 to 3 years. The number of charge cycles is also in the good number, which falls between approximately 300 to 500 cycles. This also depends on how often we use the batteries, how carefully they are used, and the temperature.
The life of a battery is the period up to which the battery delivers power until it is drained out. A charge cycle determines the period from which the battery is fully charged, discharged, and fully recharged again.
The plot shows how the cycle life of a lithium-ion battery varies concerning the temperature.
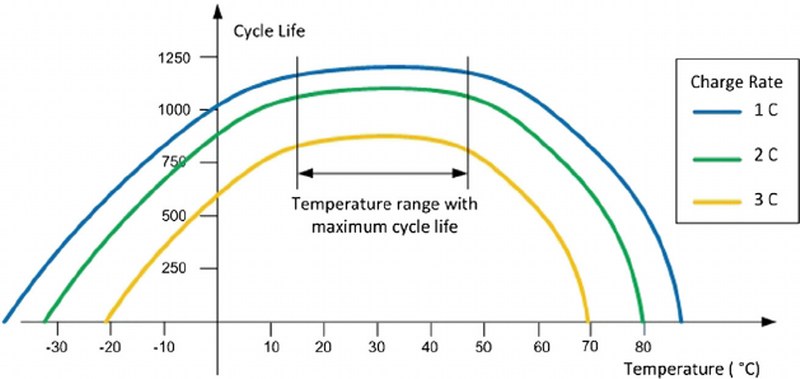
**Image courtesy: ResearchGate
How long does a Li-ion battery last on a single charge?
Once charged, it can be used for up to 8 hours. It takes only 1 hour to get fully charged. This battery does not require a cool-down period which adds to its benefits. The higher power density levels and a slower rate of capacity loss help these batteries to work for this long span.
Advantages
Almost all devices now use lithium-ion batteries. This immense hike in applications of lithium-ion batteries depicts how convenient and effective they are. Let us see the most prominent advantages of lithium-ion batteries.
- Highest energy density – 90-160 Wh/kg.
- Delivers higher nominal voltage – up to 3.6 V
- Long life – 2 to 3 years
- A large number of recharge cycles – 300 to 500 nos
- Able to withstand a wide range of temperatures
- Inexpensive, since lithium is present in naturally occurring ore.
- Non-toxic. Thus they are easy to dispose of.
- Have no memory effect – The battery does not remember the lower capacity during the repeated partial charge/discharge cycles.
- The lithium-ion batteries are comparatively of less weight. This makes them appropriate to power electrical systems in aerospace applications.
- Cause to the revolution of battery-powered cars. Thus recognized as clean energy.
Disadvantages
Like any other technology, lithium-ion batteries also face some disadvantages and limitations.
- These batteries tend to overheat. Thus thermal runaway and fire alarms may happen at high voltages.
- The mechanisms used in Li-ion batteries to limit voltage and internal pressure cause a considerable increase in battery weight and sometimes reduce battery performance.
- Costlier than Ni-Cd batteries.
- Electric vehicles are currently in high demand. Cobalt is an important element in Li-ion batteries used in these EVs. The extensive use of cobalt may cause a severe shortage in its supply.
Applications
As we have already discussed in the previous sections, the applications of lithium-ion batteries have reached uncountable. Some products exist in the market with high demand due to the advantages offered by these batteries. These are a few of the popular applications of lithium-ion batteries.
- Common electronic consumer goods like mobile phones, laptops, etc.
- Electric vehicles
- Military applications
- To power electronic devices in the aerospace sector.
- Portable medical devices that prefer longer life.
- Solar power storage
- UPS and other power backups
- Surveillance and alarm systems
- Small devices like watches that use coin batteries.
FAQs
Is it good to allow lithium-ion batteries to discharge below 95%?
No. Discharging lithium-ion batteries below 95% kills the battery prematurely. When it completely discharges, all the cobalt oxide at the cathodic end has been turned to lithium cobalt oxide. If we keep on discharging, no more lithium cobalt oxide can be formed with electrons and ions.
What happens when lithium-ion batteries are overcharged?
When the lithium-ion battery is overcharged, the electrolyte oxidizes. This generates many gases such as carbon dioxide, carbon monoxide, and methane. Carbon dioxide at the cathode increases battery temperature.
Is lithium-ion battery prone to memory effect?
No, lithium-ion batteries are not prone to memory effect, as they can be charged at any time.
In which temperature range should you operate Li-ion batteries?
The possible temperature range to operate Li-ion batteries is from 10°C to +55°C. The charging should take place at the temperature range of +5°C to +45°C.
