Last updated on April 6th, 2024 at 11:02 am
The battery has an essential function in our everyday existence. However, many of us don’t understand the basics of battery terms and characteristics. In this blog post, we will discuss the different characteristics of batteries and explain some common battery terminology. We will also provide tips to help you keep them in optimum condition. So, let’s get started
Table of Contents
Fundamental Terminologies
Let us start with some basic terminologies of the battery. Though, there may be a chance that you might know them very well, what would be a better way to revise the terms?
Anode
An anode is the negative terminal of a battery. It releases electrons to the load and thus performs reduction. This is the normal case where the battery discharges. If it charges, the anode accepts electrons and acts as a positive terminal.
Cathode
A cathode is the positive terminal. It accepts electrons and thus performs oxidation. This is the normal case where the battery discharges. If it charges, the cathode releases electrons to the load and acts as a negative terminal.
Cell

A cell is a single unit that stores electrical energy. It has positive and negative terminals and an electrolyte. When it is connected to a circuit, a chemical reaction happens through the terminals and the electrolyte to discharge electrical energy.
Battery
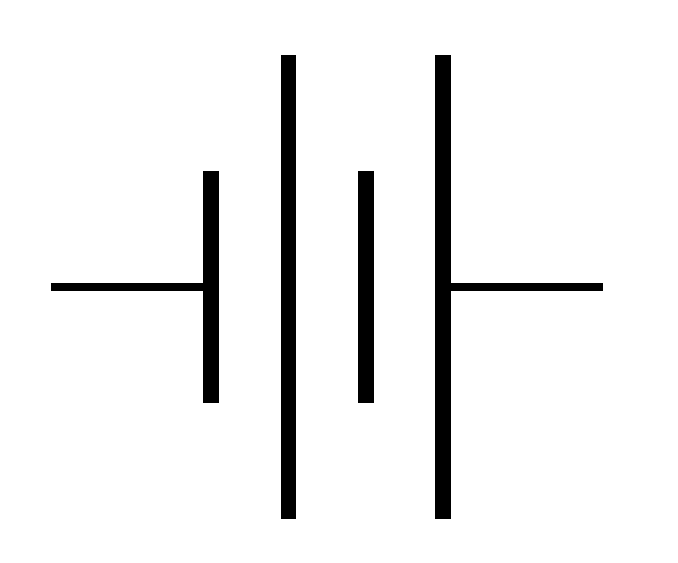
A battery is a group of two or more cells connected in a specific pattern(either series or parallel) that depends on the purpose. Read more on battery construction.
Terminals
A device is connected to an external circuit at its terminals. Every device has a positive and negative terminal.
Electron
An electron is a negatively charged particle in an atom. It revolves around the nucleus of the atom. The electrons are released, acquired, or shared during the chemical reactions.
Electrode
An electrode is a conductor through which electrons flow in a cell to produce electric energy. Every cell has a positive and negative electrode.
Electrolyte
An electrolyte is a solution of chemical compounds that facilitates ions to react with the cathode and anode.
Current
Current is the rate of flow of electrons. It is expressed in Ampere(A).
Voltage Drop
A voltage drop is a net difference in potential when measured across two ends of a conductor. It is expressed in volts (V).
Energy
It is the amount of power released. In the case of a battery, the energy is the amount of electrical energy released. The equation to find the energy of a cell is
E = Voltage x Discharge Rate x Discharge Time
Cell energy is usually expressed in milli-watt hours (MWh).
Terms Related to Battery Operation
Now let us discuss some terms related to the operation of a battery. It also includes the terminologies that mention various stages of battery operation.
Accumulator
A rechargeable battery is technically called an accumulator.
Ambient temperature
It is the average temperature of the surrounding medium. In general, higher temperatures tend to accelerate chemical reactions within, which can lead to increased power output. However, if the temperature gets too high, it can cause it to overheat and become damaged.
Conversely, low temperatures can slow down chemical reactions and reduce power output. Additionally, frigid temperatures can cause the fluid to freeze, which can damage the cells. As a result, it is important to take ambient temperature into account when operating a device powered by a battery.
Capacity

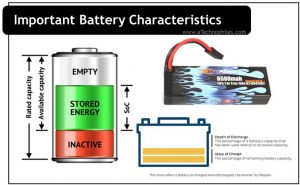
The capacity refers to the amount of energy that it can store. This is typically measured in terms of the number of hours that the battery can power a particular device, such as a flashlight or a laptop.
The capacity of a battery is affected by several factors, including its size, its chemistry, and its design. For example, batteries with a higher capacity will typically be larger than those with a lower capacity.
Charge rate or C-Rate

The C rate is a measurement used to describe the rate at which a battery is charged or discharged relative to its capacity. It is defined as the current that will discharge or charge the battery in one hour if it is discharged or charged at that rate.
For example, if a battery has a capacity of 1,000 milliamp-hours (mAh), a C-rate of 0.5 would indicate that the current draw is 500 mA or half the capacity of the battery. A C-rate of 2 would indicate that the current draw is 2,000 mA or double the capacity of the battery.
Constant current charge
It is the process of charging where the current is maintained constant throughout regardless of the voltage of a cell. Constant current charging is typically used for fast-charging applications, as it allows for a higher rate of charge than constant voltage charging.
There are several advantages to constant current charging, including improved safety and a lower risk of overcharging. However, it is important to note that constant current charging must be carefully monitored, as it can damage batteries if not done correctly.
Constant voltage charge
It is the process of charging where the voltage applied is maintained constant throughout regardless of the current drawn. This technique is often used for lead-acid batteries, as it helps to extend their lifespan by preventing them from being overcharged.
The main disadvantage of constant voltage charging is that it can take longer to charge a battery than other methods, such as constant current charging. However, this method is generally considered to be safer for both batteries and chargers, making it a good choice for many applications.
Corrosion

Corrosion is the chemical process that leads to the degradation of battery terminals. Uncontrolled corrosion results in battery failure. To prevent corrosion in batteries, manufacturers use several different materials and coatings, such as zinc and chromate.
However, even with these precautions, it is still important to regularly inspect batteries for signs of corrosion and to clean them if necessary.
Cutoff voltage
Cut-off voltage is the minimum voltage that a power source can be safely discharged to without damaging it. Cutoff voltage is also referred to as End-of Discharge voltage. Most power sources have a cut-off voltage of around 2.5 volts per cell, which means that a power source must not be discharged below 2.5 volts multiplied by the number of cells in the power source.
For example, a typical AA power source has three cells, so the cut-off voltage would be 2.5 x 3 = 7.5 volts. Once a power source is discharged below its cut-off voltage, the chemical reaction that generates electricity will stop and the power source will be unable to hold a charge. Therefore, it is important to ensure that power sources are never discharged below their cut-off voltage.
Cycle life
A battery’s cycle life is the number of times it can be discharged and recharged before it reaches the end of its useful life. For most consumer applications, a battery’s cycle life is typically between 300 and 500 cycles. Lithium-ion batteries, however, can last up to 1,500 cycles. The cycle life of a battery is directly related to its depth of discharge (DoD).
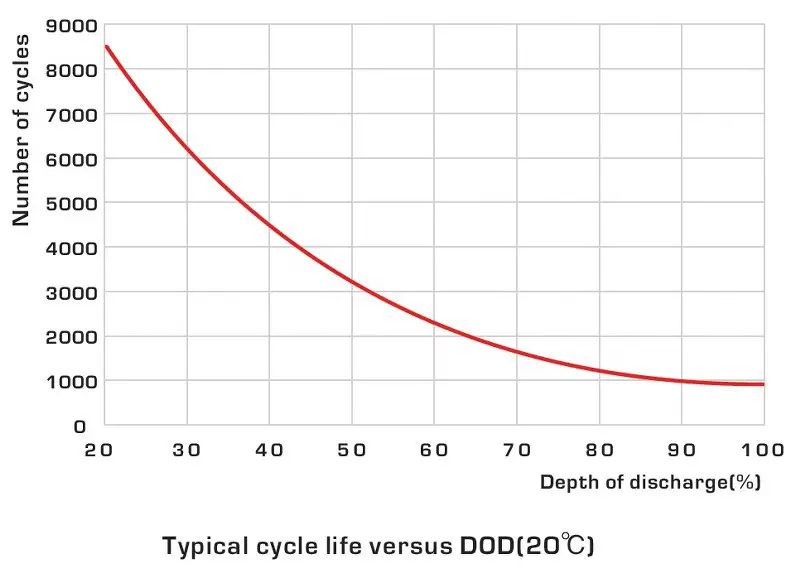
Deep cycle
A deep cycle refers to the ability to repeatedly discharge and recharge without damaging the power source. The depth of discharge (DOD) measures how much charge has been utilized relative to its full capacity.
For instance, if a 100 amp-hour (Ah) system is discharged by 50 amps, the DOD would be 50%. Systems capable of enduring numerous deep cycles will exhibit a high DOD.
Discharging
Discharging is the process by which the battery releases its electrical energy to the circuit.
Energy density
Energy density is the volumetric estimation of energy stored in a battery. It is the ratio of cell energy to its volume. Several factors contribute to the energy density of a battery, including the type of electrodes used, the electrolyte used, and the size and shape of the cell.
Internal resistance
Internal resistance is the resistance offered by the ionic and electronic components of the cell. Internal resistance can be decreased by using higher-quality materials in the construction of the battery, and by keeping the battery’s internal components cool.
Limiting current
The term “limiting current” refers to the maximum amount of current that can be drawn from a battery without damaging it. This limit is set by the battery’s internal resistance, which regulates the flow of current between the battery’s positive and negative terminals.
When the limiting current is exceeded, the battery’s internal resistance increases, causing the battery to overheat and potentially explode.
Oxidation
The chemical reaction that results in the release of electrons is known as oxidation.
Primary Cell
Non-rechargeable cells are called primary cells. The alkaline and zinc-carbon cells are primary cells.
Reduction
The chemical reaction that acquires electrons is known as reduction.
Secondary cell
Secondary cells are rechargeable cells. Most devices including mobile phones use secondary cells.
Self-discharge
When batteries are unused for a long period, the amount of energy that a battery can store begins to decline, and eventually, it will no longer be able to power devices. One way to prolong the life of a battery is to keep it stored in a cool, dry place. This will slow down the self-discharge process and allow the battery to be used for a longer period.
Another way to reduce self-discharge is to use devices that are designed specifically for low-power applications. These devices draw less current from the battery, which reduces the amount of energy that is lost over time.
Separator
The separator is insulation in the cell that keeps the anode and cathode separate.
Service life
Service life is the useful lifetime of a battery until it hits its cutoff voltage.
Shelf life
The shelf life of a battery is the maximum duration in which the battery can be stored in the preferable conditions so that it retains its performance when used. Read more
Specific energy
It is the amount of energy that can be stored in a battery per unit of mass (in units of watt-hours per kilogram, or Wh/kg). The higher the specific energy, the more energy a battery can store for a given mass.
Trickle charge
Trickle charge is a low-rate charging of a battery with a constant-current supply to maintain the battery in a fully charged condition.
Tips to keep a battery in optimum condition
If you want to keep your battery in optimum condition, there are a few things you can do.
Don’t discharge completely. This can damage and shorten its lifespan.
Avoid overcharging. It can cause the electrodes to degrade over time, which reduces the capacity of the battery.
Keep the battery clean and free of debris. This will help to prevent the build-up of corrosion.
Avoid extreme temperatures. Extreme cold or heat can also damage your battery. When it’s cold, the battery drains faster and doesn’t charge as well. When it’s hot, the battery can overheat and become damaged.