Last updated on April 6th, 2024 at 11:28 am
Batteries are one of the most important inventions in history. They store energy that can be used to power devices like cell phones, laptops, and cars. But do you know how does a battery work? In this blog post, we’ll explore how batteries work, their types, and their basics. We’ll also discuss some of the applications of batteries. But before that, let’s look at the history of batteries.
Table of Contents
History of Batteries
Today the batteries are available in various shapes and sizes. But, the initial developments were bulky and much different from the current ones. Have a look at some of the initially developed batteries.
The wet cells
Benjamin Franklin in the 18th century first created a setup that worked like a battery. In his setup, the electrodes were dipped in a jar containing an electrolyte. This setup was named the “Wet cells”.
The first true battery

With time, attempts were made to improve the performance. Alessandro Volta came up with a design that performed a chemical reaction to store and release the charges. This arrangement then came to be known as “Voltaic Pile”.
Limitations
This model exhibited several limitations among which the corrosion of zinc plates was predominant. The Voltas battery went through several stages of evolution which improved its structure as well as performance. The corrosion of electrodes remains unsolved but now with certain techniques, this process can be delayed.
New models
Voltaic Pile’s immediate successor was the Trough Battery and the journey went on. Now the type of batteries varies in size and models range from city clock batteries to heavy solar panels. Batteries are available in a wide range of capacities and technology that suits the purpose.
What is a cell?
A cell is a technical term for a single energy source that emits energy from a chemical reaction. This points to the fact that what we casually call a battery, is technically a cell. A cell is manufactured with a predefined volume of energy.

The requirement for more energy is achieved by combining multiple cells in series. This combination of cells is technically called batteries.
What is a battery?
A battery is a combination of cells that stores energy in the form of chemical energy. It is available in different capacities according to which it can be used to power devices. The chemical reaction that happens in batteries causes the electrons to flow.

Batteries can power devices up to a limit that depends on the amount of energy stored and the load connected. The simple batteries used in households, which are usually called alkaline batteries power small devices. Once it drains out, it is thrown out. However reusable batteries are available on the market and can be recharged after use.
Types of battery
Batteries vary in shape, size, capacity, and usage. As already mentioned, batteries are available as both rechargeable and non-rechargeable.
- In small devices like watches, tiny round batteries are present (aka Coin cells)
- In devices like toys and remotes, cylindrical batteries are used (aka AA batteries)
Larger capacity batteries are used in laptops and other electronic devices.
What is the difference between a cell and a battery?
The battery is a conventional method of addressing these storage devices. The actual terminology for its single piece is ‘cell’. A cell is a single unit that provides electrical energy from the stored chemical energy. A battery is a collection of cells connected in any fashion to transfer energy.
Thus it is comparatively bulky and is capable of storing more energy. So it is used to transfer energy for heavy loads. Perfect examples are the power bank and the inverters used in our houses. Even a 9 Volt battery is made up of six 1.5 V cells.
Note: For ease of understanding, this article uses the term ‘battery’ to address the energy storage unit.
Cell and battery symbol
The symbol in the figure below is used to denote the presence of a battery or a cell in the circuit.

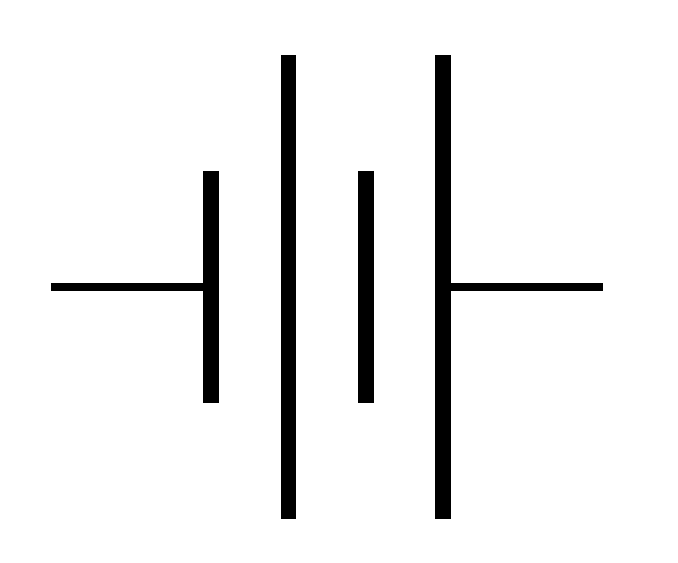
The short line denotes the negative terminal and the long line denotes the positive terminal. If only two lines are present (one short and one long) then it denotes a cell. But if multiple lines are present then it is a combination of cells, aka battery.
Components of a battery
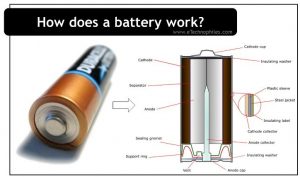
The three major components are cathode, anode, and electrolyte. A cathode is a positive terminal, an anode is a negative terminal and an electrolyte is a chemical substance that reacts with the terminals. The battery is connected to the circuit at these terminals.
These components are arranged carefully to ensure the perfect progress of chemical reactions as well as to ensure perfect insulation.
Structure
Let us discuss the basic structure of an ordinary alkaline battery, as shown in the figure below:

The outer plastic covering wrapped tight around the battery contains all the primary information such as the cell’s capacity, voltage, terminals, etc. It also has a key role in providing insulation.
Anode & Cathode identification
The plain end of an alkaline battery is the anode, while the popped-out region (the other end) is the cathode. Under the outer insulation, another covering of steel and nickel plating is present. It prevents the internal components from interacting with external atmospheric factors.
Multiple layers of different materials are present in the battery. These are placed in such a way that certain levels of voltage and current are maintained during the chemical reaction.
How does a battery work?
A battery consists of different materials made of atoms. A controlled reaction occurs inside the battery where the atoms interact with each other which causes free mobile electrons.
Reaction at anode
The anode is made of zinc. The atoms in electrolytes combine with the zinc atoms and undergo oxidation. As a result, free electrons are released which get collected under the brass pin.
Reaction at cathode
The cathode is made of manganese oxide. The atoms in the electrolyte combine with the manganese oxide atoms and undergo reduction.
Accumulation of electrons
As electrons are negatively charged, the accumulation of electrons under the brass pin makes it a negative terminal, while the other end becomes a positive terminal. This creates a potential difference at the two ends of the battery.
Movement of electrons
The free electrons tend to move towards the positive terminal. But, due to a separator between both terminals, this movement does not occur. Hence, when the battery is not connected to any circuit, no electron movement happens.
When it gets connected to a circuit, it creates a path for the electrons to reach the positive terminal. Hence a conventional current starts flowing, opposite to the direction of electron flow. In this way, a battery works in a circuit.
Applications
The invention of batteries revolutionized our lives. Almost all the devices that we use now contain a type of battery inside it. Though the applications make a long list, let us see some of the prominent ones.
- In houses, we use batteries to power remote controls, torchlight, etc.
- In our day-to-day life, rechargeable batteries are used in digital cameras, cell phones laptops, etc.
- Batteries are also used in health instruments such as hearing aids, valve-assisting devices, artificial limbs, etc.
- The batteries are also used in the medical sector. A particular example is an ECG heart monitor which should be kept ON all the time. Lithium-ion and nickel batteries are mostly utilized for such purposes.
- The battery acts as a safety backup that can be used where there is a fluctuating power supply or unavailability of power.
- Batteries in wireless receivers and radios for Military operations are another vital application.
- One of the most celebrated applications of batteries is in the case of electric vehicles. Lithium-ion batteries are used to power automobiles that promote pollution-free as well as cost-effective transport.