Last updated on April 5th, 2024 at 05:14 pm
A battery is an important element of the electronics sector. Among them, rechargeable batteries are the most commonly used batteries today. The lead acid battery was one of the first rechargeable batteries.
The chances of reusing these batteries and reduction in their disposal were seen as a very effective milestone in the evolution of batteries. This article gives you the essential details about lead acid batteries.
Table of Contents
History
The lead acid batteries were introduced in the year 1859 by Gaston Plante. It is one of the oldest rechargeable batteries, the first available for commercial use. This secondary battery thus had a huge acceptance in the market.
Since then, lead-acid batteries have been used in most rechargeable battery applications. They are highly utilized in photovoltaic systems and in starting car engines.
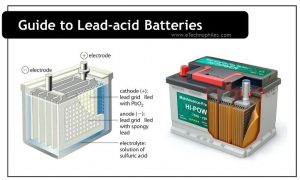
Construction of a Lead acid battery
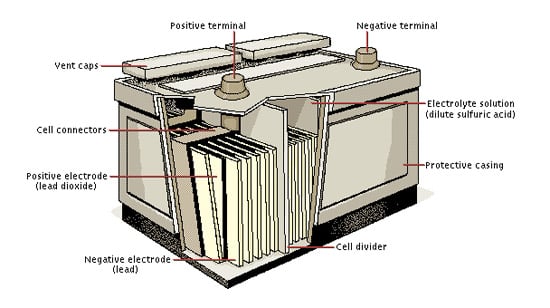
A lead acid battery consists of six cells of 2.0V coupled together. Thus the battery provides an overall voltage of 12.0V. These cells are mounted side-by-side in a single case and sealed together. This arrangement makes them bulky. But still, they are sometimes preferred over lead acid batteries due to their ability to deliver large voltage.
Lithium-ion batteries are currently the strong competitor of lead-acid batteries. While Li-ion battery technology is highly used to drive cars, lead acid batteries still exist as the best choice to start the car engine and power the other automobile segments.
Battery chemistry
Let us discuss the structure and working of lead-acid batteries.
Battery structure
Lead is the major component present in lead acid batteries. The anode is made of spongy lead and the cathode is made of lead oxide. The porous and soft lead is used in the anode to ease the dissolution of lead. The metals like “lead-antimony” and “leadcalcium.” are added to the anode to increase electrode strength.

These electrodes are dipped in the electrolytic solution of sulfuric acid and water. A chemically permeable membrane is placed in between the electrodes to prevent direct contact. It also prevents electrical shorting through the electrolyte. The battery is covered with a thick rubber or plastic case to prevent leakage of the corrosive sulphuric acid.
Battery discharging process

The lead and lead oxide react with sulfuric acid. This reaction results in the formation of lead sulfate crystals at both electrodes, as well as releases free electrons. The lead sulfate uses sulfate from the sulfuric acid and reduces its concentration resulting in the formation of lead sulfate and water.
The full discharge results in the loss of potential between the electrodes. Thus it is not a good practice to discharge this battery completely.
Battery charging process
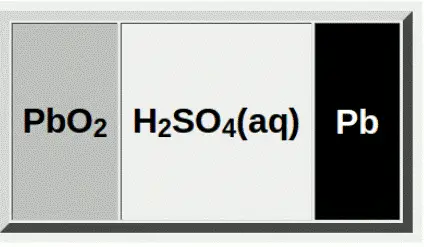
The lead acid batteries start charging if we provide a voltage above 2.1V. As with every rechargeable battery, the charging reverses the chemical reaction in the batteries and brings the electrodes and electrolytes back to their composition.
Note: A constant voltage supply is mandatory to charge a lead-acid battery.
The typical charging time of a lead acid battery is around 12 to 18 hours. Larger batteries take even more time up to 36 hours.
Advantages
Lead acid batteries are very popular in the category of secondary batteries. It has been extensively used in numerous applications these days. Here are the most relevant advantages of lead-acid batteries which made them a highly accepted choice.
- The lead acid batteries provide a comparatively higher voltage of 12.0V. Thus they can be used in high current drain applications.
- They are highly cost-effective in terms of cost-per-watt basis.
- They perform the best in cold temperatures, even in subzero conditions.
- It is a convenient choice for automobiles since it powers every stage from starting to electronics and much more.
- Lead acid batteries have easy manufacturing stages with relatively low technology equipment.
- They are not subjected to the battery memory effect like that in Nickel batteries which reduces the battery capacity.
- They are available in numerous sizes and specifications.
- They are tolerant of the damages due to overcharging.
- There are numerous producers of Lead acid batteries worldwide. Thus, they are easily available.
Disadvantages
Apart from this long list of advantages, lead acid batteries face some disadvantages that resulted in replacing them in several applications. Some of them are included here.
- Their most prominent limitation is that it is heavy and bulky. It thus consumes lots of space.
- The full discharge of lead acid batteries results in reducing their capacity.
- It requires a long charging time of 14-16 hours to get fully charged. Thus they are not suitable for fast charging.
- It has a relatively short cycle life due to the corrosion of electrodes.
- Their overall efficiency is just around 70% and has a low energy density.
- These batteries should be disposed of with care since lead is not environmentally friendly.
- These batteries are subject to high maintenance requirements.
- They offer a small energy-to-volume ratio and a very low energy-to-weight ratio.
Applications
Lead-acid batteries are used in numerous applications to utilize the advantage of rechargeable batteries. Some of them are replaced with modern technologies like lithium-ion batteries. But Lead acid batteries are still the perfect choice in numerous other applications. Some of them are listed below.
- The most common application of lead acid batteries is to start the automobile engine. It is a perfect choice since the they can work in even low temperatures.
- Lead-acid batteries are used to power automobile accessories like headlights and other lamps since they produce a larger voltage (12V).
- They are used to power electric parts in conventional submarines when submerged because they are the best to perform in low temperatures.
- They were widely used in battery-powered electric vehicles like electrified bicycles, wheelchairs, electric scooters, etc.
- They are largely used in UPSs as the backup power supply.
FAQs
Is lead-acid battery repairable?
Yes, they can be repaired in some cases by replacing individual cells or through maintenance procedures like cleaning and equalization charging, but severe damage may render them irreparable.
What is the voltage of each cell in a lead-acid battery?
Each cell has a voltage of 2.0V in a lead-acid battery.